valence electrons for br|How many valence electrons does brom : Baguio 119 rows — Mar 23, 2023 — For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence . corporate website: atalian.com EUROPE: BELGIUM BOSNIA HERZEGOVINA CROATIA CZECH REPUBLIC FRANCE HUNGARY LUXEMBOURG NETHERLANDS POLAND ROMANIA RUSSIA SERBIA SLOVAKIA TURKEY ASIA: BURMA
PH0 · Valences of the Chemical Elements
PH1 · Valence Electrons Chart for All Elements
PH2 · How to Find the Valence Electrons for Bromine (Br)
PH3 · How many valence electrons does bromine have?
PH4 · How many valence electrons does brom
PH5 · How many valence electrons does Brom
PH6 · How Many Valence Electrons Does Bromine (Br) Have? [Valency of Bro
PH7 · How Many Valence Electrons Does Bromine (Br) Have?
PH8 · How Many Valence Electrons Does Bromine (Br)
PH9 · How Many Valence Electrons Does Bro
PH10 · Complete Electron Configuration for Bromine (Br, Br
PH11 · Bromine Valence Electrons
PH12 · Bromine Electron Configuration (Br) with Orbital Diagram
PH13 · Bromine Electron Configuration (Br) wit
PH14 · Bromine (Br)
Game One Piece vs Fairy Tail 2.0 – Chơi game One Piece vs Fairy Tail 2.0 đánh nhau đối kháng hai người online mới nhất trên game vui 24h kbh 4399 snokido poki tro choi net Thể loại: game hoat hinh, game hanh dong Giới thiệu game One Piece vs Fairy Tail 2.0. Game One Piece vs Fairy Tail 2.0 là một trò chơi đánh nhau đối kháng cực hot và nổi .
valence electrons for br*******119 rows — Mar 23, 2023 — For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence .Set 2, 2020 — There are two ways to find the number of valence electrons in Bromine (Br). The first is to use the Periodic Table to figure out how many electrons Bromine has in its valence shell. .
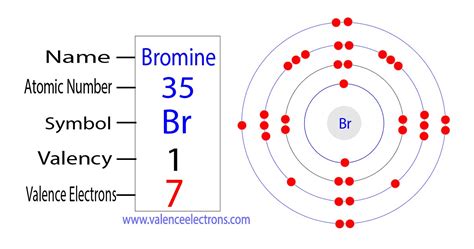
Peb 3, 2021 — The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). Thus, bromine has .
93 rows — May 19, 2024 — You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at .Peb 1, 2021 — The diagram breaks down the total numbers of Bromine valence electrons of atoms. Dot diagram further helps in understanding the interaction of valence electrons. It draws the numbers of dots around the symbol of Br as .
Mar 18, 2023 — The electron configuration of bromide ion(Br –) shows that the bromide ion acquired the electron configuration of krypton. Bromine atom exhibit -1, +1, +3, +5 oxidation states. The oxidation state of the element changes .
Peb 1, 2021 — Bromine Valence Electrons. Valence electrons can be found in the p and S highest energy orbitals. Br has an electron configuration of1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. The valence electrons are there in the 4s and 4p .
Red volatile liquid at room temperature. Its reactivity is somewhere between chlorine and iodine. Harmful to human tissue in a liquid state, the vapour irritates eyes and throat. Discovered in .Ene 16, 2023 — Electrons going into d sublevel can play either a role of valence electrons or shielding electrons. So there is not always a certain number of apparent valence electrons. The number of apparent valence electrons for the first transition metal period is shown in the table below. Under construction. Figure 2: Valence electrons for transition metals.
Ago 14, 2020 — The chlorine atom has the same electron configuration in the valence shell, but because the entering electron is going into the n = 3 shell, it occupies a considerably larger region of space and the electron–electron repulsions are reduced. The entering electron does not experience as much repulsion and the chlorine atom accepts an additional .The electrons that determine valence – how an atom reacts chemically – are those with the highest energy.. For a main-group element, the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n. [1] Thus, the number of valence electrons that it may have depends on the electron configuration in a .The presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: For a main group element, a valence electron can only be in the outermost electron shell. An atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be chemically .valence electrons for brMar 24, 2021 — Steps for Writing Lewis Structures. Example \(\PageIndex{2}\) 1. Determine the total number of valence electrons in the molecule or ion. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(2)(1) + 4 + 6] = 12 valence electrons. 2.valence electrons for br How many valence electrons does bromMar 24, 2021 — Steps for Writing Lewis Structures. Example \(\PageIndex{2}\) 1. Determine the total number of valence electrons in the molecule or ion. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(2)(1) + 4 + 6] = 12 valence electrons. 2.Abr 17, 2023 — Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. The number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. For the main group elements (groups designated with a .May 1, 2024 — Find your element on the table. Now, locate the element that you want to find the valence electrons for on the table. You can do this with its chemical symbol (the letters in each box), its atomic number (the number in the top left of each box), or any of the other pieces of information available to you on the table.. For example purposes, let's find the valence .May 19, 2024 — This table of element valences includes the maximum valence and most common valence values in chemistry. Use this for reference with a periodic table. . While these are the most common valences, the real behavior of electrons is less simple. Remember an element's electron cloud will become more stable by filling, emptying, or half-filling the .Bromine is the 35th element in the periodic table and has a symbol of Br and atomic number of 35. It has an atomic weight of 79.904 and a mass number of 79. Bromine has thirty-five protons and forty-four neutrons in its nucleus, and thirty-five electrons in four shells. It is located in group seventeen, period four and block p of the periodic .
The Octet Rule. The other halogen molecules (F 2, Br 2, I 2, and At 2) form bonds like those in the chlorine molecule: one single bond between atoms and three lone pairs of electrons per atom.This allows each halogen atom to have a noble gas electron configuration, which corresponds to eight valence electrons.

The Octet Rule. The other halogen molecules (F 2, Br 2, I 2, and At 2) form bonds like those in the chlorine molecule: one single bond between atoms and three lone pairs of electrons per atom.This allows each halogen atom to have a noble gas electron configuration, which corresponds to eight valence electrons.How many valence electrons does bromThe Octet Rule. The other halogen molecules (F 2, Br 2, I 2, and At 2) form bonds like those in the chlorine molecule: one single bond between atoms and three lone pairs of electrons per atom.This allows each halogen atom to have a noble gas electron configuration, which corresponds to eight valence electrons.Valence electrons in a Bromine atom: The atomic number of Bromine Br is 35. The electronic configuration of Bromine Br can be written as 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5; The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a total of seven .
For the main group elements, the valence electron exists only in the outermost electron shell. A valence electron can exist in the inner shell of a transition metal. An atom consisting of a closed shell of valence electrons will usually .May 25, 2023 — Now let’s see how you can easily find the valence electrons of Bromine atom (Br). If you don’t want to read the texts, then you can also watch this video. How to find the Valence Electrons? (2 Methods) In order to find the valence electrons of a Bromine atom (Br), you can use two methods.Ago 20, 2021 — Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) sublevel are called inner-shell electrons and are not involved directly in the element's reactivity, or in the formation of compounds. Lithium has a single electron in the second principal .
May 6, 2018 — 7 only the electrons in the outmost shell are valance electrons.All but seven of the electrons in bromine are in lower shells Bromine is in family VII A. the same as Fluorine Chlorine. All members of the family have seven valance electron hence the name 7A.Drawing the Lewis Structure for BrO 3-. Viewing Notes: The BrO 3-Lewis structure has a total of 26 valence electrons. This includes the electron represented by the negative charge in BrO 3-.; You need to put brackets around the BrO 3-Lewis structure as well as a negative charge to show that the structure is a negative ion.; If you calculate the formal charges for the initial BrO 3 .
Nob 16, 2015 — The "Br"^(-)" ion has 36 electrons. The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. A neutral bromine atom would also have 35 electrons. In order for a bromine atom to become a 1- bromide ion, it would have to gain an additional electron. Below is the Lewis dot structure for a neutral bromine atom, which has .Total electron pairs are determined by dividing the number total valence electrons by two. For, Br 2 molecule, Total pairs of electrons are seven in their valence shells. Center atom of Br 2 molecule. Because, there are only two atoms and both atoms belongs to same element, do not need to worry about center atom selection. .
La nouvelle adresse du site Cpasbien mise à jour régulièrement. La dernière adresse accessible du site est cpasbien[.]news. L’impact des sites de torrent sur l’industrie cinématographie. T’as une idée du nombre de vidéos piratées visionnées chaque année ? Plus de 230 milliards! Rien qu’aux États-Unis, c’est 15 milliards de .f you’ve just wrapped up watching the latest Hindi Prime Video sitcom, then here’s how things unfolded during the Happy Family Conditions Apply Season 1 Ending.Starring Ratna Pathak Shah as .
valence electrons for br|How many valence electrons does brom